Which of the Following Is a Strong Acid
Which one of the following is a strong acid. H2SO3aq 1 ionized C.

Solved Which Of The Following Species Are Strong Acids Chegg Com
HNO2aq 1 ionized D.

. B HI and HBr. Thus it is a strong acid. A CH 3 COOH B HNO 3 C H 2 CO 3 D All of the above Medium Solution Verified by Toppr Correct option is B Acids which ionizes completely into its ions are called strong acids.
September 29 2020September 29 2020essayfalconessayfalcon0 Comments. Citric acid acetic acid tartaric acid are organic acids. Questions in other subjects.
Each of the following pairs contains one strong acid and one weak acid except. The correct option is D Hydrochloric acid. Buffer _____ is a measure of the ability of a buffer to maintain the pH following the addition of strong acid or base.
Sulfuric acid H₂SO₄ and hydrochloric acid HCl are both of them very strong acids. Which of the following options correctly describe the behavior of this system when a strong acid is added to it. Write a description of alpha decay for po-.
E HClO2 H C l O 2. D H2CO3 H 2 C O 3. After each decay press the reset nucleus button to watch the process again.
Hence among the given options hydrochloric acid is a strong acid. Acid that ionises completely in aqueous solution thus producing a high concentration of H 3. C The Moon orbits the Earth in the same length of time that it takes for the Earth to orbit the Sun.
A eqHNO_3eq This compound is Nitric Acid a. A Previous question Next question. Select all that apply-The ratio HAAHAA- will increase-The overall pH will decrease only slightly.
Nitric acid dissociates completely in its aqueous solution. Correct option is A Nitric acid also known as aqua fortis is a highly corrosive mineral acid. E HBr and H3PO4.
The substance CH3CH22NH is considered to be A. Nitric acid HNO3 is classified as a strong acid in water. When a solution of hydrochloric acid HCL reacts with zinc which of.
C H2SO4 and H2S. Jo is at the gym one afternoon jogging on the. A HNO2 B H29 C H2SO4 D KOH E NaBr F NaOH G HCI H H2CO3 1 NH3 J KC2H2O2 Which of the following is a weak acid.
Start an single atom tab. It is fully dissociated in diluted solution except in extremely acidic solutions. Which one of the following is a strong acid.
Which of the following is a strong acid. During reaction both neutralise each other to form a neutral NaCl. HCI is a strong acid because it has more number of hydrogen ions whereas acetic acid contains less number of.
Generally organic acids are weak in nature and mineral acids are strong. A lower value of pH shows the highest strength of an acid and as the value of pH increases acidity decreases and above 7 basicity increases. From the above discussion it is clear that Acetic acid is not a strong acid.
Answer 0 cristisp93 Magnesium hydroxide Mg OH₂ is a weak base. Which one of the following is a strong acid. Which one of the following is not a strong acid.
B HI H I. C H3P O4 H 3 P O 4. Which of the following is a strong acid.
Hydrochloric acid is an example of mineral acid. This means that it produces. NaOH is a strong base and HCl is a strong acid.
H3PO4aq 1 ionized B. Analysis of the given salts. It is less concentrated than a strong acid.
A Question 5 1 point What is the pH of a solution with a H of 1 x 10-8 M. A HNO3 and H2CO3. It behaves as typical acid in.
Which of the following is a strong acid. Which of the following is a strong acid. So the correct answer is Option B.
H₃PO4 H20 OH2SO4 H2 Question 4 1 point What is the pH of a solution with a H of 1 x 10-12 M. D The Moon is stationary in the sky in relation to Earth. Here some of you may think that the higher the value of pH shows the highest strength of acidity but this is not true.
In the same length of time that it takes to make one revolution on its axis. Hence these are weak acids. Ionizes completely in solution.
A HF H F. A KHSO4 aq b HClO4 aq c H2CO3 aq d NH42C2O4 aq Expert Solution. Which of the following is a strong acid in water.
All of the Above. Which of the following is a strong acid. Observe the decay of polonium-211.
O ionsis called strong acid. Correct option is A Strong acid. The salt which is made of weak acid and strong base.
All of the Above. Answer choice A is the correct response. KNO3 is made of KOH pottasium hydroxide and HNO3.
It is a strong oxidizing agent and a strong acid at ambient temperature. 100 of the Nitric acid will have reacted with water to produce hydronium ions and NO 3 ions. A HCI hydrochloric acid Explanation.
Reacts with metals that are more active than hydrogen. Which of the following is a characteristic of a weak acid. It does not dissociate completely in water.
NaCl is made of NaOH sodium hydroxide and HCl hydrochloric acid. HNO 3 is described as a strong acid. Sodium hydroxide NaOH and potassium hydroxide KOH are hydroxides of metals from the first group in the periodic table so thy are strong bases.
It is not as dangerous as a strong acid. It does not conduct electricity in water. BProduces hydronium ions in solution.
Select all that apply A HNO2 B H25 C H2504 D KOH E NaBr F NaOH G HCI H H2CO3 1 NH3 J KC2H2O2 Question 27 1 point What is the pH of a solution with pOH of. B The Moon is stationary on its axis as it orbits the Earth. D HCl and HC2H3O2.

Which Of The Following Is A Strong Acid Neetlab

Answered Which Of The Following Is Not A Strong Bartleby

Weak Base Strong Acid Reactions Video Khan Academy
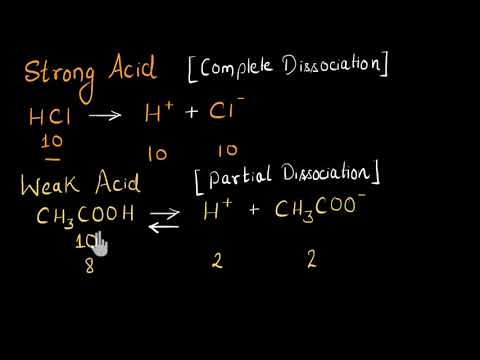
Strong And Weak Acids Bases Video Khan Academy

Solved B 14 Which Of The Following Is A Strong Acid A Chegg Com

Strong Acid Strong Base Reactions Video Khan Academy

Solved Which One Of The Following Is A Strong Acid O Nh3 Chegg Com
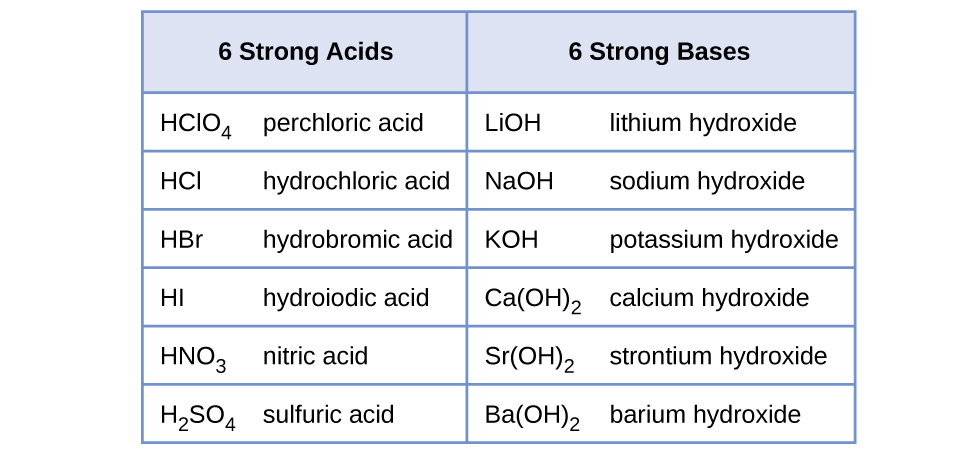
14 3 Relative Strengths Of Acids And Bases Chemistry

Solved 21 Which Of The Following Is A Strong Acid A Chegg Com

Which Of The Following Is A Strong Acid Brainly Com
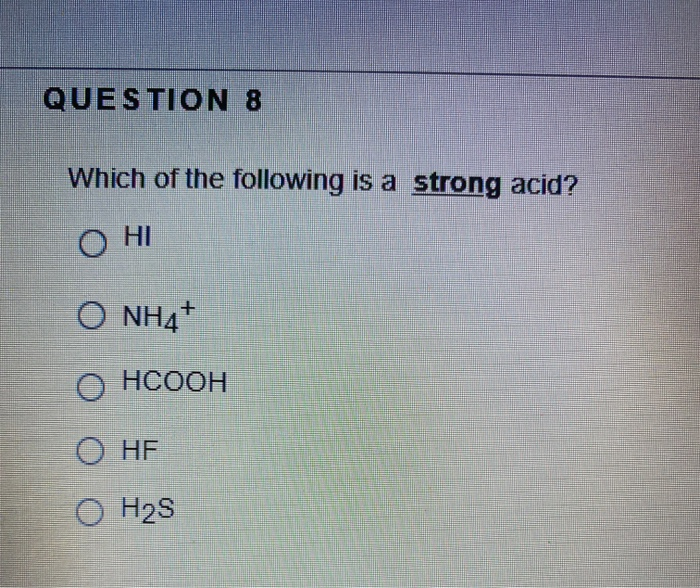
Solved Question 8 Which Of The Following Is A Strong Acid Chegg Com
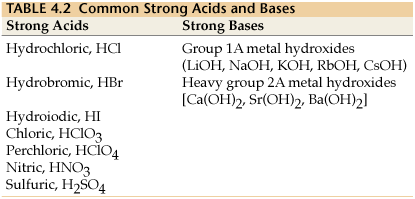
What Are Strong Acids Or Bases Are That Ionize Or Dissociate In Aqueous Solution Socratic
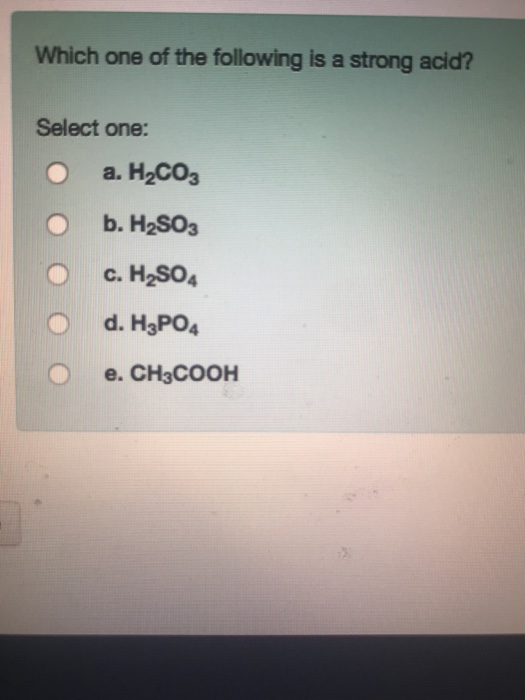
Solved Which One Of The Following Is A Strong Acid Select Chegg Com
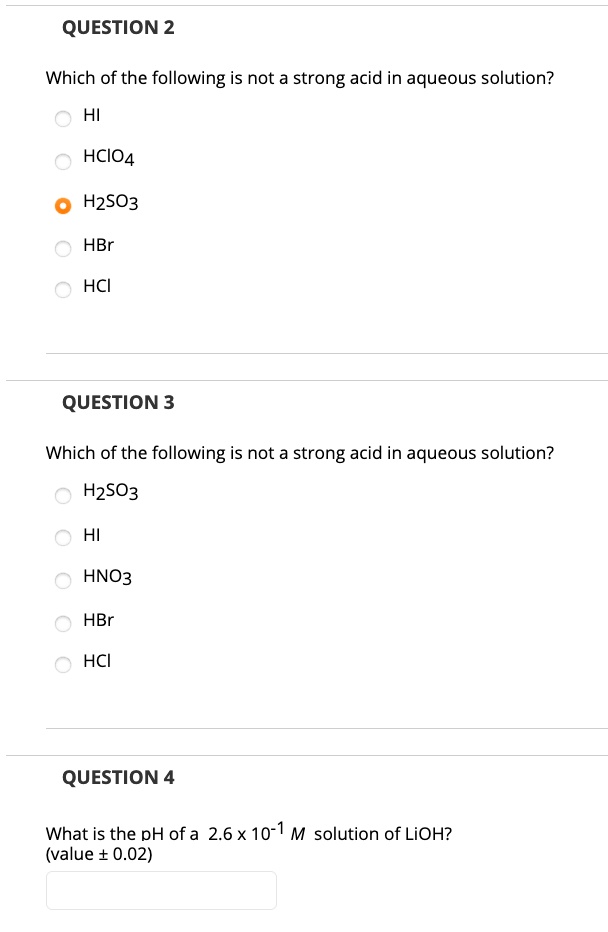
Solved Question 2 Which Of The Following Is Not A Strong Acid In Aqueous Solution Hi Hcio4 Hzso3 Hbr Hci Question 3 Which Of The Following Is Not A Strong Acid In Aqueous
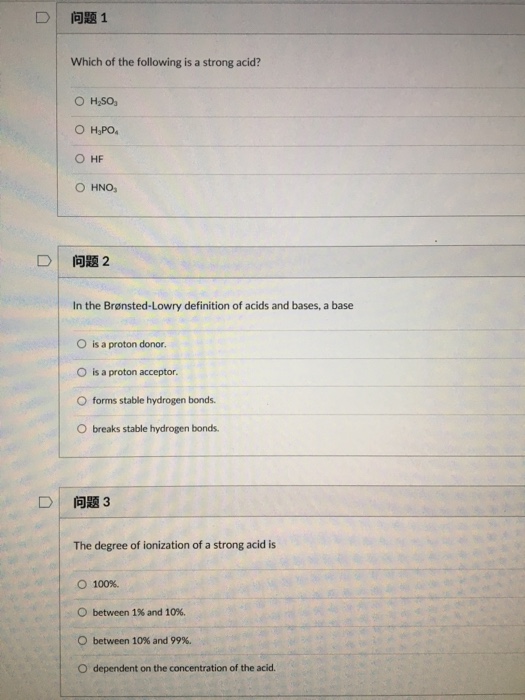
Solved D 1 Which Of The Following Is A Strong Acid Hf Chegg Com

How To Memorize The Strong Acids And Strong Bases Youtube

Which Of The Following Is A Strong Acid

Comments
Post a Comment